An Enzyme a Chemical Reaction and So It Is Ready to Perform the Reaction Again
Exploring Enzymes
A catalyzing science project
Key concepts
Biological science
Biochemistry
Enzymes
Physiology
Chemistry
Introduction
Take you lot always wondered how all the food that y'all consume gets digested? It is not only the acid in your tummy that breaks down your nutrient—many little molecules in your body, called enzymes, help with that too. Enzymes are special types of proteins that speed upward chemic reactions, such every bit the digestion of food in your stomach. In fact, in that location are thousands of unlike enzymes in your body that work around-the-clock to go on you healthy and active. In this science activity yous volition investigate one of these enzymes, chosen catalase, to find out how it helps to protect your body from damage.
Background
Enzymes are essential for our survival. These proteins, fabricated by our cells, help transform chemicals in our body, functioning as a goad. A catalyst gets reactions started and makes them happen faster, past increasing the rate of a reaction that otherwise might not happen at all, or would take too long to sustain life. Even so, a catalyst does non take office in the reaction itself—so how does this work? Each chemic reaction needs a minimum corporeality of energy to make it happen. This energy is chosen the activation energy. The lower the activation free energy of a reaction, the faster information technology takes place. If the activation energy is too high, the reaction does non occur.
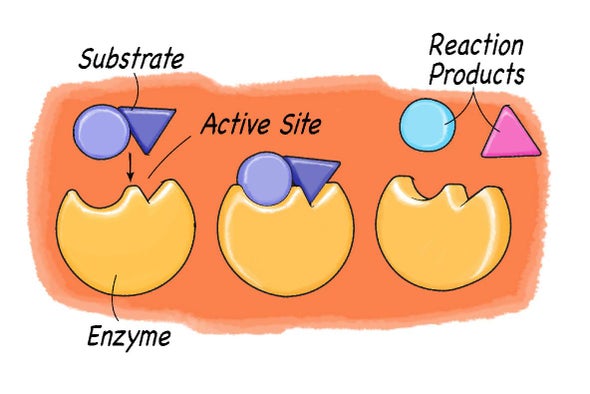
Enzymes accept the ability to lower the activation energy of a chemical reaction past interacting with its reactants (the chemicals doing the reacting). Each enzyme has an active site, which is where the reaction takes identify. These sites are like special pockets that are able to bind a chemic molecule. The compounds or molecules the enzyme reacts with are called their substrates. The enzyme pocket has a special shape so that only one specific substrate is able to bind to it, just like only one key fits into a specific lock. Once the molecule is bound to the enzyme, the chemical reaction takes place. And then, the reaction products are released from the pocket, and the enzyme is set to start all over once again with another substrate molecule.
Catalase is a very mutual enzyme that is present in near all organisms that are exposed to oxygen. The purpose of catalase in living cells is to protect them from oxidative damage, which can occur when cells or other molecules in the body come up into contact with oxidative compounds. This harm is a natural result of reactions happening inside your cells. The reactions tin can include by-products such as hydrogen peroxide, which can be harmful to the torso, only equally how a by-product of a nice bonfire can be unwanted smoke that makes you lot cough or stings your eyes. To prevent such damage, the catalase enzyme helps getting rid of these compounds by breaking upward hydrogen peroxide (H2Oii) into harmless water and oxygen. Do you want to meet the catalyze enzyme in activity? In this activity you will disarm hydrogen peroxide with the help of catalase from yeast.
Materials
- Rubber goggles or protective glasses
- V teaspoons of dish soap
- I package of dry yeast
- Hydrogen peroxide, 3 percentage (at to the lowest degree 100 mL)
- Three tablespoons
- One teaspoon
- V 16-ounce disposable plastic cups
- Tap water
- Measuring cup
- Permanent mark
- Paper towel
- Workspace that tin can get wet (and won't be damaged by whatever spilled hydrogen peroxide or nutrient-colored water)
- Nutrient coloring (optional)
Preparation
- Take one cup and deliquesce the dry yeast in about i-one-half loving cup of warm tap water. The water shouldn't be too hot just close to body temperature (37 Celsius). Let the dissolved yeast residue for at least five minutes.
- Employ the permanent mark to label the remaining 4 cups from one to four.
- To all the labeled cups, add 1 teaspoon of dish soap.
- To cup i no further additions are made at this point.
- Before using the hydrogen peroxide, put on your safety goggles to protect your eyes. In case y'all spill hydrogen peroxide, clean information technology upwardly with a wet newspaper towel. If you get it on your skin, make sure to rinse the afflicted expanse with plenty of water.
- To loving cup two, add one tablespoon of 3 percentage hydrogen peroxide solution. Use a fresh spoon for the hydrogen peroxide.
- To cup three, add two tablespoons of the hydrogen peroxide.
- To cup four, add iii tablespoons of the hydrogen peroxide.
- Optionally, yous can add a drib of food color to each of the labeled cups. (You tin can choose a dissimilar color for each one for like shooting fish in a barrel identification)
Procedure
- Take cup number one and place it in front of you on the piece of work area. With a fresh tablespoon, add one tablespoon of the dissolved yeast solution to the cup and swirl it slightly. What happens later you add the yeast? Practise yous see a reaction happening?
- Place cup number two in front end of you and again add one tablespoon of yeast solution to the cup. In one case you add together the enzyme, does the catalase react with the hydrogen peroxide? Can yous come across the reaction products existence formed?
- Add one tablespoon of yeast solution to cup number three. Do you see the same reaction taking place? Is the result different or the same compared to cup number two?
- Finally, add one tablespoon of yeast solution to cup number four. Do you run into more or less reaction products compared to your previous results? Can you explain the departure?
- Place all four cups next to each other in front of you and observe your results. Did the enzymatic reaction take place in all of the cups or was there an exception? How do the results in each cup look different? Why do you think this is the case?
- Now, accept loving cup number one and add together one additional tablespoon of 3 percent hydrogen peroxide to the cup. Swirl the cup slightly to mix the solution. What happens at present? Looking at all your results, what practice yous retrieve is the limiting factor for the catalase reaction in your cups?
- Extra: Repeat this activeness, but this time do not add dish soap to all of the reactions. What is dissimilar once you lot remove the dish soap? Exercise you lot even so meet foam formation?
- Extra: So far you take observed the outcome of substrate (HtwoO2) concentration on the catalase reaction. What happens if you keep the substrate concentration abiding but change the concentration of the enzyme? Try adding different amounts of yeast solution to three tablespoons of hydrogen peroxide, starting with ane teaspoon. Do you observe any differences, or does the concentration of catalase non matter in your reaction?
- Extra: What happens if the environmental conditions for the enzyme are changed? Repeat the catalase reaction only this time vary weather condition such every bit the pH past adding vinegar (an acrid) or blistering soda (a base), or change the reaction temperature past heating the solution in the microwave. Can you lot identify which conditions are optimal for the catalase reaction? Are there whatever conditions that eliminate the catalase activeness?
- Extra: Tin can you detect other sources of catalase enzyme that you could use in this activity? Research what other organisms, plants or cells incorporate catalase and try using these for your reaction. Do they piece of work as well equally yeast?
Observations and results
You probably saw lots of bubbles and foam in this activity. What fabricated the foam appear? When the enzyme catalase comes into contact with its substrate, hydrogen peroxide, it starts breaking it downwards into water and oxygen. Oxygen is a gas and therefore wants to escape the liquid. Nonetheless, the dish soap that you added to all your solutions is able to trap the gas bubbling, which results in the formation of a stable cream. As long as there is enzyme and hydrogen peroxide present in the solution, the reaction continues and foam is produced. Once 1 of both compounds is depleted, the product formation stops. If you do non add dish soap to the reaction, yous will see bubbling generated but no stable foam formation.
If in that location is no hydrogen peroxide present, the catalase cannot role, which is why in cup 1 you shouldn't take seen any chimera or foam production. Only when hydrogen peroxide is available, the catalase reaction tin take place every bit you probably observed in the other cups. In fact, the catalase reaction is dependent on the substrate concentration. If y'all have an excess of enzyme just not enough substrate, the reaction will be limited by the substrate availability. In one case you lot add more than hydrogen peroxide to the solution, the reaction rate volition increase as more substrate molecules tin collide with the enzyme, forming more product. The result is an increasing amount of cream produced in your cup equally yous increment the amount of HtwoO2 in your reaction. Y'all should have seen more foam being produced once you added another tablespoon of hydrogen peroxide to cup ane, which should have resulted in a like corporeality of foam as in cup ii. However, at some point you will attain a substrate concentration at which the enzyme gets saturated and becomes the limiting factor. In this example you have to add more enzyme to speed upwardly the reaction once again.
Many other factors impact the activity of enzymes equally well. Most enzymes only function under optimal ecology atmospheric condition. If the pH or temperature deviates from these conditions as well much, the enzyme reaction slows downward significantly or does not piece of work at all. You might take noticed that when doing the actress steps in the process.
Cleanup
Pour all the solutions into the sink and make clean all the spoons with warm water and dish soap. Wipe your work surface area with a moisture paper towel and wash your easily with water and lather.
More to explore
Biological science for Kids; Enzymes, from Ducksters
Enzymes: The Trivial Molecules That Bake Bread, from Scientific American
Catalase, from PDB-101
Enzyme-Catalyzed Reactions—What Affects Their Rates?, from Science Buddies
The Liver: Helping Enzymes Assist You lot!, from Scientific American
Science Action for All Ages!, from Science Buddies
This action brought to you in partnership with Scientific discipline Buddies

Source: https://www.scientificamerican.com/article/exploring-enzymes/
0 Response to "An Enzyme a Chemical Reaction and So It Is Ready to Perform the Reaction Again"
Post a Comment